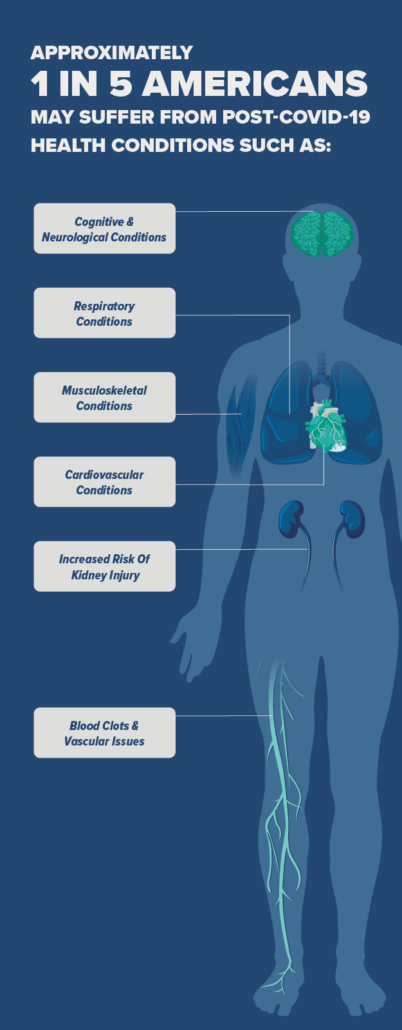
The peak of the pandemic may have passed, but for tens of millions of Americans, their fight against Covid-19 continues. According to the CDC, as many as one in five Americans may suffer from Long Covid. Those figures are replicated pretty much everywhere in the world.
Millions suffer the same fate globally: fatigue, brain fog, migraines, tremors, chronic pain, anxiety, depression, and more, often lasting more than three months since the initial infection. Many cannot work or do the same activities with their families they enjoyed before the pandemic.
This illness, characterized by a diverse collection of symptoms, continues to baffle researchers. The cause is difficult to ascertain, and some patients have been known to experience up to 200 different symptoms. There is currently no universally recognized clinical definition for Long Covid and lack of consistent terminology about the disease itself further hinders diagnosis and scientific understanding.
Virginia Amann of ENMEDIA says, “While many of us recognize the name Long Covid, others refer to it as Long-haul Covid, Post-Covid, Post-Acute Sequale of Covid-19 (PASC) or Chronic Covid. This lack of consistency in Long Covid terminology hinders communications between groups and stifles progress in the field.”
While many of us recognize the name Long Covid, others refer to it as Long-haul Covid, Post-Covid, Post-Acute Sequale of Covid-19 (PASC) or Chronic Covid. This lack of consistency in Long Covid terminology hinders communications between groups and stifles progress in the field.
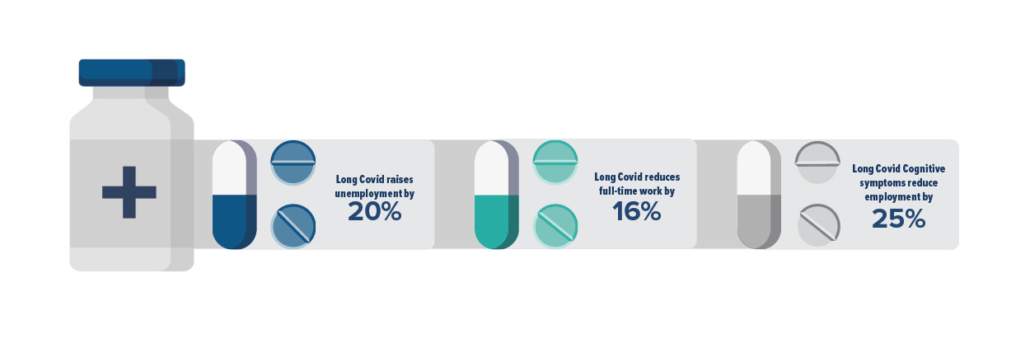
The economic impact of Long Covid is immense. David Cutler at Harvard University has estimated the economic impact of Long Covid to be $3.7 trillion. Research conducted in February this year found that Long Covid was associated with 23% higher odds of being unemployed and 16% lower odds of working full-time. The latter figure was 25% for those with cognitive symptoms. It’s also a strain on overworked healthcare systems, still reeling from the pandemic and labor shortages, which now must deal with a new problem — one that we currently have no answer to.
Progress Addressing Long Covid
Despite the widespread and urgent nature of this problem, progress in addressing it has been remarkably slow, particularly when we compare it to the historic speed at which Operation Warp Speed developed working vaccines against the virus itself.
Investigators working on the NIH’s dedicated Long Covid initiative — Researching Covid-19 to Enhance Recovery (RECOVER) — have since warned that “hospital systems and healthcare providers currently lack the tools to address Long Covid. For example, there are no current best clinical practices for providing care to individuals with Long Covid, and the national response toward developing such practices lacks coordination.”
In 2020, Congress invested $1.2 billion into researching and treating Long Covid through the National Institutes of Health — a huge sum of money, by any account.
Goals of RECOVER Initiative
The goals of RECOVER are threefold: to define the clinical spectrum and pathophysiology of Long Covid, to determine its natural history and prevalence, and to characterize the way in which Covid-19 causes post-acute sequelae, or long-term health effects.
But the NIH has faced criticism for the glacial rollout of trials or other research aimed at dealing with Long Covid — especially given the huge amount of congressional funding it has received. Patients, meanwhile, have pleaded with authorities to do more research into Long Covid and help them.
Patient advocacy groups such as the Patient-Led Research Collaborative have been active participants in Long Covid research initiatives, but they feel like the process lacks urgency and has failed to produce any meaningful results.
Lisa McCorkell, a Long Covid patient and researcher said, “There’s no treatment specific for Long Covid. It was clear from the Covid pandemic that we have the capacity to invest into a large response for therapeutics and drug development generally, so why are we not doing that for long Covid?”
Insights obtained through the RECOVER Initiative
That said, RECOVER has uncovered some valuable findings. For example, research published in February documented how Black and Hispanic Americans are more likely than White Americans to suffer from some of the symptoms that characterize Long Covid, but they are less likely to be diagnosed.
While these findings are an important step, they offer little immediate relief to those currently suffering from Long Covid and seeking to regain some semblance of their pre-infection lives.
Research on Long Covid has been slow, focusing on symptoms that are incredibly variable between patients and not enough on underlying causes. Many trials also focus on already developed treatment options that have already been adequately researched instead of developing new therapeutics and diagnostics specifically for Long Covid. Meanwhile, the number of Long Covid patients continues to grow with no clear treatment options on the horizon.
While the NIH’s RECOVER program has trundled along at a perhaps lackluster pace, the private sector has been ramping up its own response to Long Covid — and the results are promising.
Private Sector Response and Promising Findings
When it comes to Long Covid, HealthBio, an emerging company founded by Dr. Bruce Patterson formerly Associate Professor of Pathology and Infectious Diseases and Director of Virology at Stanford University, is a key leader in the field. They found that the prolonged presence of viral S1 protein within non-classical monocytes after Covid-19 infection is associated with Long Covid. Patterson and his team believe these monocytes make their way to blood vessels and contribute to chronic inflammation and vascular tissue injury.
As for treatment, HealthBio’s approach holds promise. The company is investigating the combination of maraviroc (used to treat HIV infection) and pravastatin, which targets fractalkine, as a potential therapeutic approach. Early results from clinical trials are promising across a range of Long Covid symptoms.
After the introduction of maraviroc and pravastatin, “participants showed a decrease in modified Rankin scale scores [measuring disability and dependence in daily activities] and reported improvement in neurological function and ability” — simply put, their neurological symptoms eased and they could function better. That was mirrored in their physical symptoms, too. They could handle more physical exertion, and improvement in respiratory functions as observed.
Another way HealthBio’s research is working to mitigate this harm is by developing ways to predict severe instances of Covid-19 and those more likely to result in Long Covid. Their research into the immunological landscape of Covid-19 yielded meaningful results. Specifically, they used machine learning methods to identify at-risk individuals from other classes by analyzing cytokines – small proteins released by cells that have a specific effect on the interactions, communication or behavior of cells. Cytokines play essential roles in the body’s immune system and inflammatory responses.
HealthBio said: “These models, which can be incorporated into clinical laboratory information systems, enable a highly accurate, immune-based classification of severe Covid-19 infection and Long Covid. This workflow will greatly aid the triage, treatment, and prognosis of those affected.”
Other companies, too, are moving forward. Pfizer has been researching and developing the use of antiviral medicine Paxlovid soon after infection to avert Long Covid.
Paxlovid is already designed to alleviate serious cases of Covid-19 and to lower the chances of hospitalization or death in at-risk patients. But now, data suggests it could be used across the board with patients to minimize the risk of Long Covid.
A study published this year in the journal JAMA Internal Medicine found that people who took Paxlovid within five days of a positive Covid-19 test saw a 26% lower risk of Long Covid compared with those who didn’t receive it. More than 35,000 people took the oral Covid pill in the study, while 246,000 did not.
In July of this year, enrollment began for the RECOVER-Vital trial, which will evaluate Paxlovid as a potential treatment for Long Covid.
Data Tracking
The Veterans Health Administration is becoming an important place of healthcare innovation for Long Covid patients. That’s because it tracks symptoms, treatment, and outcomes across its system of 23 Covid facilities. The organization can share data and findings internally, and track lots of patients, which is typically challenging within the fragmented US healthcare system.
The Broader Long Covid Innovation Ecosystem
Despite some criticism, RECOVER appears to be an important part of a wider, US and global effort to deal with Long Covid. It is — albeit slowly — building up a body of research and giving academic institutions and some researchers a direction and much-needed funding. It is planning and recruiting for trials.
There is, of course, room for improvement. More and faster-moving engagement with the private sector would undoubtedly add value to the ecosystem at large. RECOVER may not have been the all-encompassing, dynamic, and near-miraculous mobilization that Operation Warp Speed was, but it does appear to be a large central cog in the wider Long Covid innovation ecosystem.
In this ongoing battle against Long Covid, the collective efforts of numerous players such as the NIH’s RECOVER initiative, private sector companies, and health institutions, have begun to unveil promising pathways towards understanding and combating this enigmatic condition.
Their collaboration reinforces the global commitment to health and recovery, setting the stage for the advancements yet to come.
As the adage goes, the largest cogs may turn the slowest, but every rotation brings us closer to the solutions we urgently need.
